Dextrose Equivalent (DE) is a key metric in the world of food science, giving insight into the amount of reducing sugars in a carbohydrate product and their impact on properties like sweetness, viscosity, and browning. DE indicates how extensively starch molecules break down into simpler sugars, affecting everything from texture to shelf stability. Understanding DE is essential for formulators across industries, as the right DE level can tailor products to specific functional needs.
In this blog, we’ll unpack DE’s role, its effects on food and drink, and how it’s measured, offering a clear look into its importance in ingredient science.
What is Dextrose Equivalent (DE)?
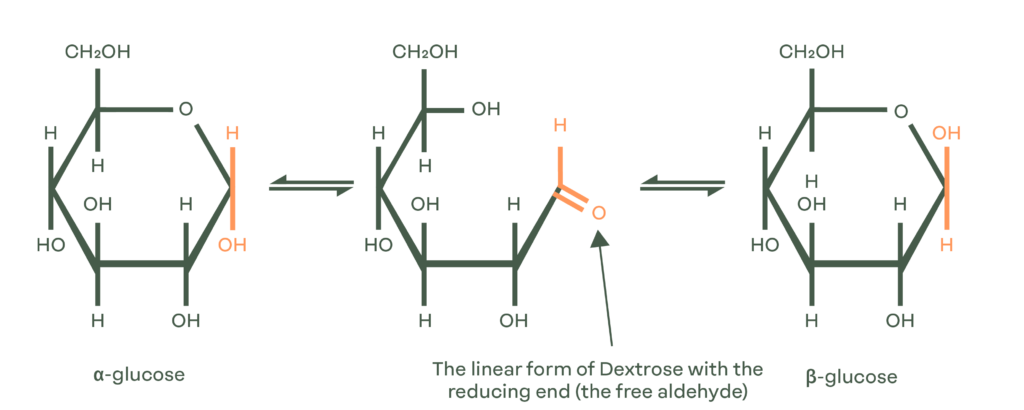
Dextrose Equivalent (DE) is a measure of the amount of reducing sugars present in a sugar, or carbohydrate. A reducing sugar contains a free aldehyde group (an organic compound containing a functional group), which allows it to act as a reducing agent. DE is expressed as a percentage on a dry basis relative to dextrose, where the presence of water is overlooked for the purposes of the calculation.
Dextrose is otherwise known as d-glucose, an isomer (a type of molecule) that is found in nature, and is used to distinguish it from l-glucose which is not. Glucose refers to both d-glucose and l-glucose. The dextrose equivalent gives an indication of the average degree of polymerisation (DP) for starch.
Polysaccharides (such as Starch) are linked by glycosidic bonds. When these bonds are broken, they are reduced to smaller molecules like monosaccharides (one saccharide) or disaccharides (two monosaccharides bonded). In the human body when we consume carbohydrates, we break these bonds through a chemical reaction known as hydrolysis, which allows the molecules consumed to be prepared for metabolism (energy production). In food technology, the exact same chemical reaction is used to break the bonds of carbohydrates and create specific sugars!
Some examples of carbohydrates would be:
- Glucose (Monosaccharide)
- Fructose (Monosaccharide)
- Maltose (Disaccharide)
Dextrose equivalent is typically measured by titration (Lane Eynon or Luff Schrool). Using titration, product is added to react with the aldehyde group. As an example with Dextrose, the test then measures the shift from the alpha or beta cyclic forms to the linear form. The DE of chemically pure Dextrose is 100 as in its linear form, it has a free aldehyde group.
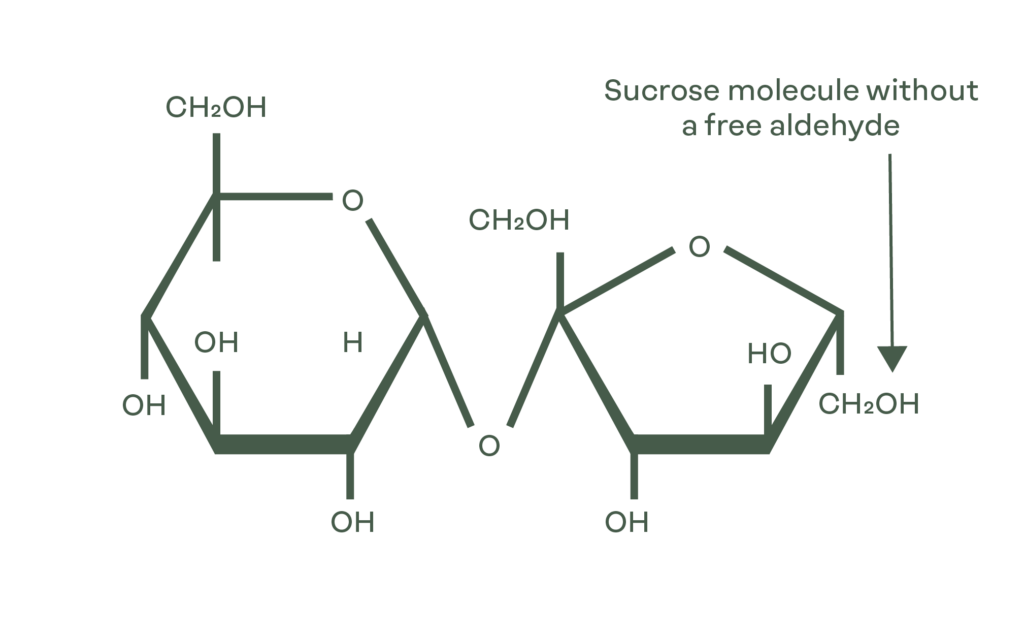
Uniquely, the DE of sucrose is zero because there isn’t a free aldehyde.
Dextrose Equivalent and Sweetness
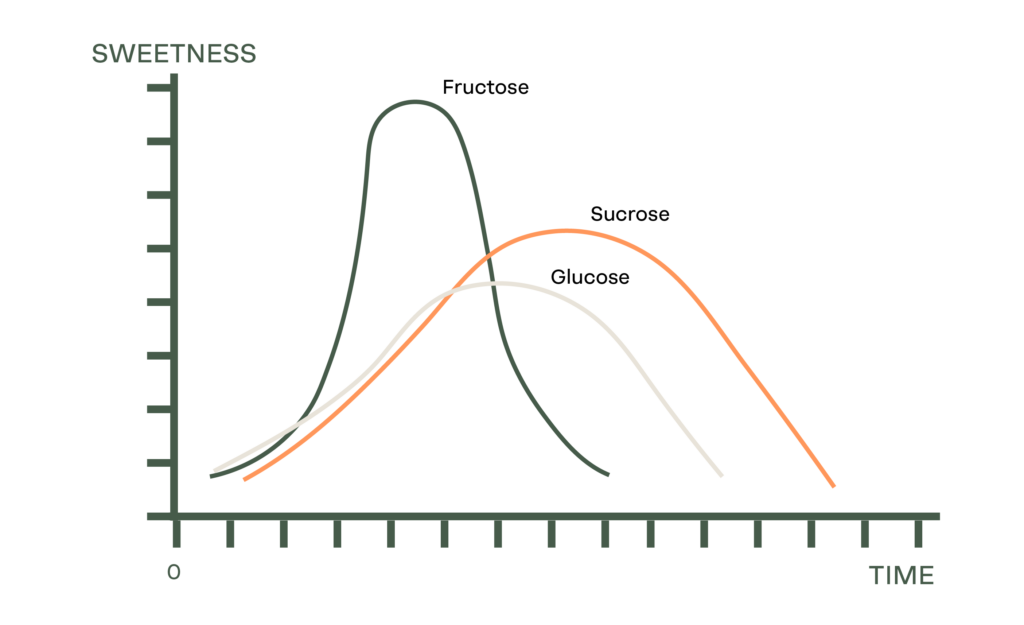
A common misconception is that DE equates to the sweetness of a syrup. While a higher DE can correlate with a high sweetness, that is not what it stands for. As a non-scientific explanation, a 0-30DE Glucose Syrup is roughly correlated to sweetness. Outside of this range however, sweetness cannot be predicted using the Dextrose Equivalent. Relative sweetness (that is compared typically to Sucrose as 1) should be assessed as well as considering the time intensity of a sweetener (which will impact the consumer taste perception).
Degree of Polymerisation (DP)
The degree of polymerization (DP) is defined as the number of monomeric units in the polymer linked by glycosidic bonds. Dextrose and fructose have a DP of 1. Maltose has a DP of 2.
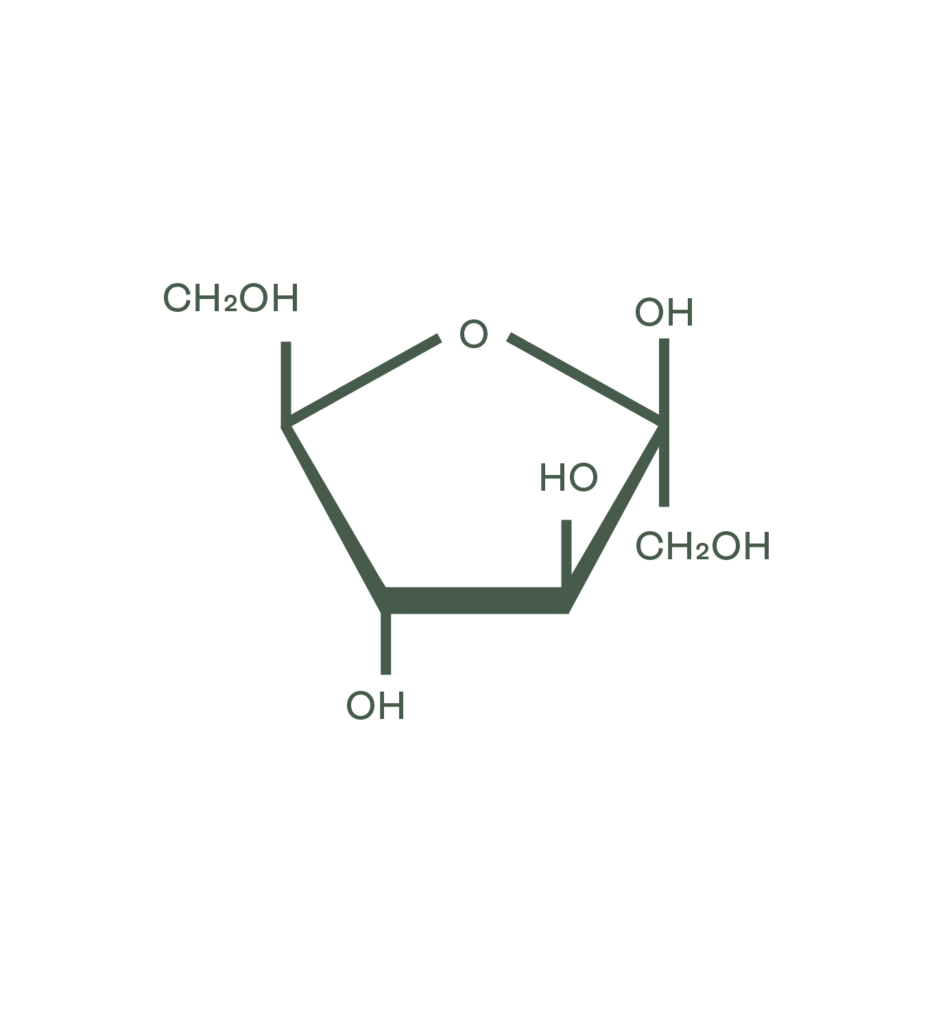
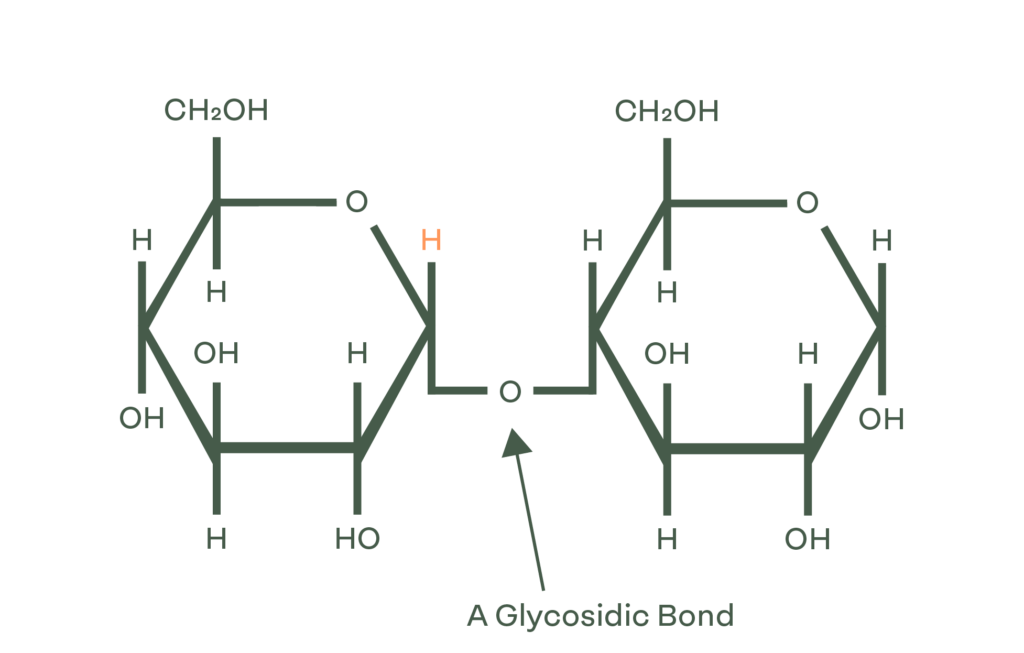
Carbohydrates are classified by their degree of polymerization (DP). The term ‘sugars’ refers to the sum of all the mono- and di-saccharides (DP1 & DP2). Higher DPs are not classified as sugars, but as carbohydrates. It is important to note that while sugars are distinguished separately from carbohydrates, sugars are a subcategory of carbohydrates.
- Mono-saccharides (DP1): Simple sugars with only 1 sugar unit, such as glucose (d-glucose or l-glucose), fructose and galactose.
- Di-saccharides (DP2): Contain 2 sugar units linked to each other by a chemical bond, such as sucrose (1 glucose and 1 fructose), maltose (2 glucose units), and lactose (1 glucose and 1 galactose).
- Tri-saccharides (DP3): Composed of 3 bound sugar units, such as maltotriose (3 glucose units).
- Oligosaccharides (DP10-): Contain up to 9 sugar units bound to each other.
- Polysaccharides (DP10+): Contain 10 or more sugar units bound to each other. These are referred to as complex carbohydrates (such as starch).
| DP | Carbohydrate | DE |
|---|---|---|
| 1 | Dextrose & Fructose | 100 |
| 2 | Maltose | 52.6 |
| 3 | DP3 | 35.7 |
| 4 | DP4 | 27 |
| 5 | DP5 | 21.7 |
| 6 | DP6 | 18.2 |
| 7 | DP7 | 15.6 |
| 8 | DP8 | 13.7 |
| 9 | DP9 | 12.2 |
| 10 | DP10 | 11 |
| 2000000 | Starch | 0 |
As you can see above, the higher the polymerization, the lower the DE. Pure dextrose and fructose have a DE of 100 while starch has nearly a DE of 0.
Sugar Spectrum
A detailed understanding of the sugar spectrum is needed to fully understand the Dextrose Equivalent, as each molecule contributes differently to the DE. The sugar spectrum can be understood as the makeup of the different carbohydrate molecules present. Below you can see a few examples of DE contribution.
| Type | DE | Fructose | Dextrose | Maltose | Maltotriose | Higher Sugars |
|---|---|---|---|---|---|---|
| Low Converted Glucose | 30 | 5 | 11 | 14 | 70 | |
| Very high Converted Glucose | 96 | 95 | 3 | 1 | 1 | |
| Acid Converted Glucose | 38 | 17 | 13 | 12 | 58 | |
| High Dextrose High Maltose | 65 | 37 | 30 | 9 | 24 | |
| High Dextrose High Maltose | 55 | 25 | 37 | 9 | 29 | |
| High Maltose | 43 | 5 | 52 | 17 | 26 | |
| Very High Maltose | 53 | 3 | 70 | 12 | 15 | |
| Glucose-Fructose Syrup “F9” | 60 | 9 | 35 | 30 | 10 | 25 |
| Glucose-Fructose Syrup “F42” | 96 | 42 | 53 | 3 | 1 | 1 |
| Fructose-Glucose Syrup “F55” | 96 | 55 | 41 | 3 | 1 | 1 |
| Dextrose | 100 | 100 | ||||
| Fructose | 100 | 100 |
What is the Influence of DE on Functionality?
DE greatly influences the functional properties of a carbohydrate, and a formulator will select one based upon what properties they are trying to achieve in their formulation.
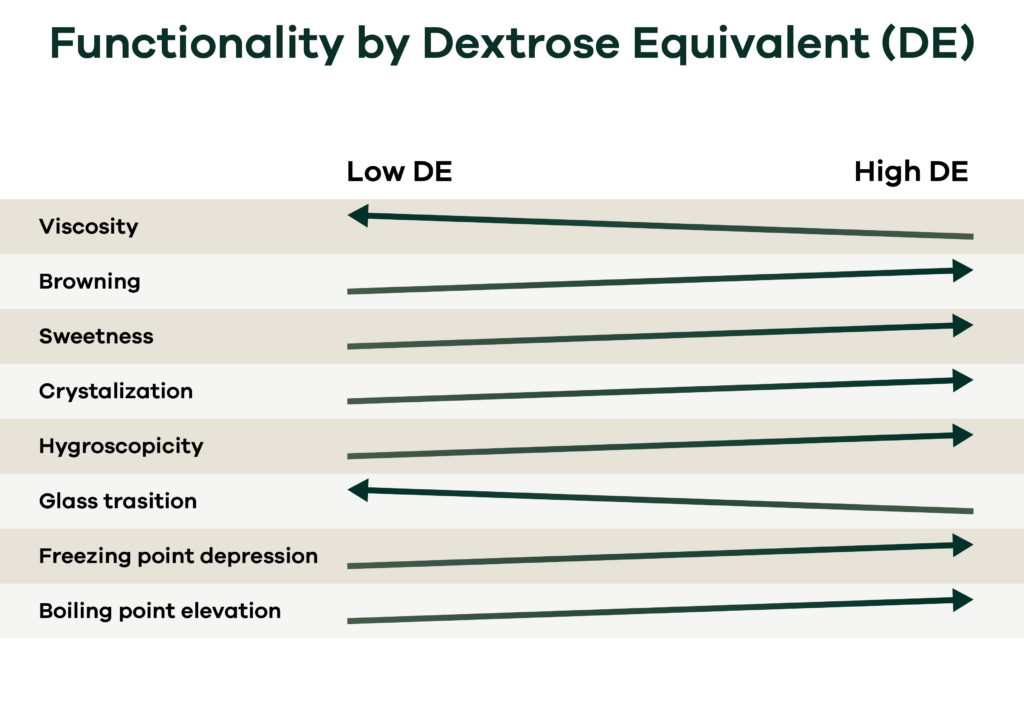
You will recall that Fructose and Dextrose both have a DE of 100. However, the functionality of Fructose and Dextrose are different due to Fructose being more in an open form which is more reactive than Dextrose. Fructose will increase the sweetness, browning and hygroscopicity. The glass transition temperature will also be considerably lower than dextrose. Therefore, the DE cannot be used as an absolute quantification of the functionality of the syrup.
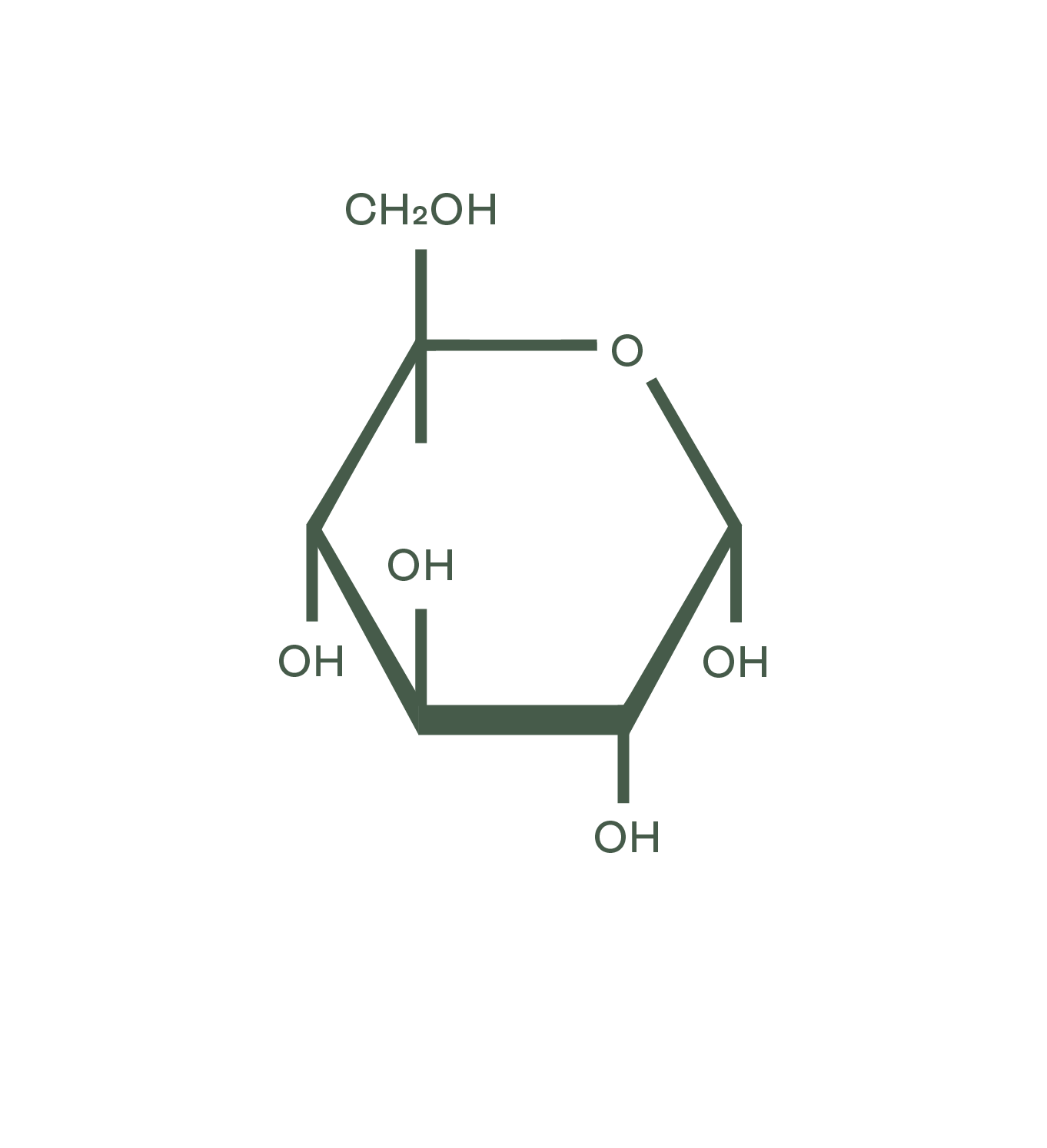
Dextrose molecular structure
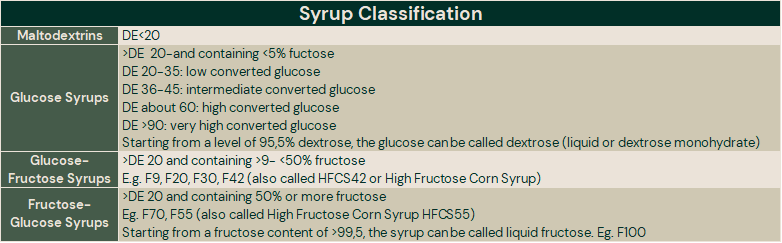
Syrup Classifications
Frequently Asked Questions (FAQs)
1. Does DE represent the sweetness of a carbohydrate?
The sweetness of a carbohydrate is not the same as Dextrose Equivalent. When exploring sweetness, a ‘relative sweetness’ to sucrose (considered to be 1) should be used. For example, chemically pure Fructose and Dextrose both have a DE of 100. Fructose however is 1.17 x the sweetness of sucrose, whereas Dextrose is 0.75 x the sweetness of sucrose.
2. How can I find out the DE of a carbohydrate?
Ask the manufacturer of the carbohydrate or review the product specification.
3. Does the DE of a carbohydrate affect the functionality in a recipe?
Functionality and DE are heavily correlated. Knowing whether a carbohydrate is a low or high DE will allow us to understand the general functionality. However, this is not absolute as functionality can be influenced by factors not related to DE.
References
Wikipedia contributors. (2024a, July 8). Reducing agent – Wikipedia. https://en.wikipedia.org/wiki/Reducing_agent
Wikipedia contributors. (2024b, August 26). Hydrolysis. Wikipedia. https://en.wikipedia.org/wiki/Hydrolysis
Wikipedia contributors. (2024c, October 15). Polysaccharide. Wikipedia. https://en.wikipedia.org/wiki/Polysaccharide
Wikipedia contributors. (2024d, October 29). Carbohydrate. Wikipedia. https://en.wikipedia.org/wiki/Carbohydrate
Wikipedia contributors. (2024e, October 24). Reducing sugar – Wikipedia. https://en.wikipedia.org/wiki/Reducing_sugar
Wikipedia contributors. (2024f, November 12). Sugar – Wikipedia. https://en.wikipedia.org/wiki/Sugar
Wikipedia contributors. (2024g, March 8). Dry basis – Wikipedia. https://en.wikipedia.org/wiki/Dry_basis
Wikipedia contributors. (2024h, November 15). Dextrose – Wikipedia. https://en.wikipedia.org/wiki/Glucose#Chemical_and_physical_properties
Wikipedia contributors. (2024i, April 7). Degree of polymerization – Wikipedia. https://en.wikipedia.org/wiki/Degree_of_polymerization
Contributors: Alain Destexhe (ADM)
How can we help?
At Lehmann Ingredients, our 35 years of experiences in sourcing and supplying top-tier ingredients makes us a trusted partner for businesses of all sizes, both in the UK and internationally. Our expertise in sweeteners and ingredients has helped industry leaders and startups alike bring quality and innovation to their products. If you’re looking to enhance your formulations with reliable, high-quality sweeteners, our team is here to help. Reach out to us at enquiries@lehmanningredients.co.uk or call +44 (0) 1524 581560 to explore our full portfolio and discover how we can support your ingredient needs.




